
Projects
Our aim is to elucidate how synaptic dysfunction in neurodevelopmental disorders alters the development of functional networks at the cellular scale. Our approach integrates tools from neurophysiology, neuroanatomy, stem cell biology, genomics, computational neuroscience, applied math, engineering and computer science. We are always looking for team members and collaborators to join us in investigating network development at the cellular scale.
Current research
Effect of Mecp2 deficiency on functional connectivity in developing cortical networks
Loss of Mecp2, the cause of Rett syndrome and some cases of autism, leads to a devastating decline in cognitive, motor and sensory function
in infancy. Mecp2-deficient mice also show a postnatal decline in behavioral, motor and sensory function. Investigations at the synaptic level reveal that loss of Mecp2 has
opposing effects on the development of excitatory and inhibitory neuronal populations (Mierau et al, Biol Psych, 2015 epub). To understand how these synaptic changes alter
network development at the cellular scale, we are using microelectrode array (MEA) recording and two-photon calcium imaging of spontaneous network activity in Mecp2-deficient and wild-type
cortical cultures. We used methods from network science, including graph and control theory, to understand the effects on the
topology of the network and roles of individual neurons in the network activity over early development in vitro. We are also investigating network dynamics to determine whether there
are changes in the network topology and/or nodal roles on the seconds-to-minutes scale our recordings. This research will help us understanding how information processing is impaired at the cellular scale
and provide a cellular-scale platform for testing novel therapeutic strategies. Our collaborators include:
Bianca Dumitrascu, Stephen Eglen, Ole Paulsen, Manuel Schroeter, Tim Sit, and Sinead Williamson. This work has been supported by the
American Academy of Neurology (S.M.) and Medical Research Council DTP (A.D.).
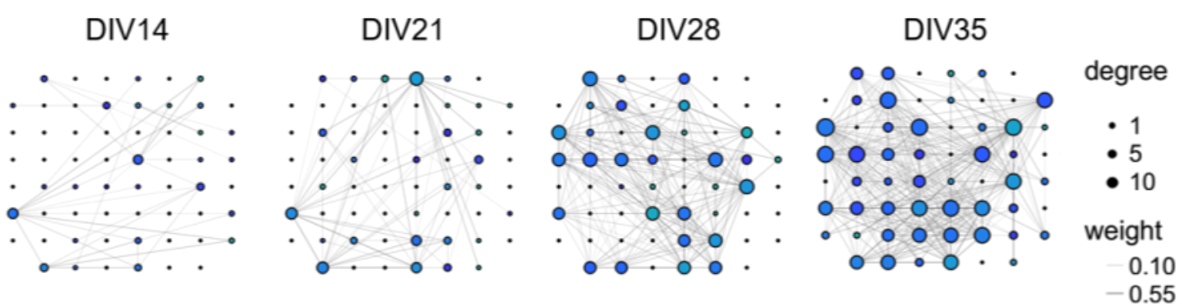
Image: Graphs show development of functional connectivity in a cortical network cultured on an MEA.
Network function in a human cerebral organoid model of Rett syndrome Human cerebral organoids offer a human-derived cellular platform for investigating underlying mechanisms of neurologic disease and testing novel therapeutic strategies. From our studies in Mecp2-deficient mice, we know that network development is altered. Through an interdisciplinary collaboration with Andras Lakatos, Madeline Lancaster, and Ole Paulsen, we are generating novel Mecp2-deficient air-liquid interface cerebral organoids (ALI-COs). We will use these organoids to study network dysfunction in human-derived cortical networks and how this may contribute to epilepsy and cognitive dysfunction in Rett syndrome. This work is supported by the NIH. It was previously supported by the Evelyn Trust. Image: MEA recording from an ALI-CO and network plot we made for Giandomenico, et al. (2019) Nat Neurosci (see published collaborations below).

Novel cell-type specific mediators of NMDA receptor maturation We have combined laser capture microdissection and single cell RNA sequencing to identify novel regulators of synpatic NMDA receptor development with cell-type, layer- and region-specificity in the cortex. We are validating our findings with spatial transcriptomics. This research is important for understanding the mechanisms and for modulating the development of excitatory synaptic transmission with cell-type specificity. This will provide novel targets for treating Rett syndrome and other neurologic and psychiatric disorders in which NMDA receptor dysfunction is implicated. Our collaborators include: Martin Hemberg, Jimmy Lee, Ole Paulsen, and Omer Bayraktar. This work was suppored by the European Commission Horizons 2020 MSCA (S.M.) and the Wellcome Trust Sanger Institute (M.H., O.B.).
Microscale network analysis pipelines
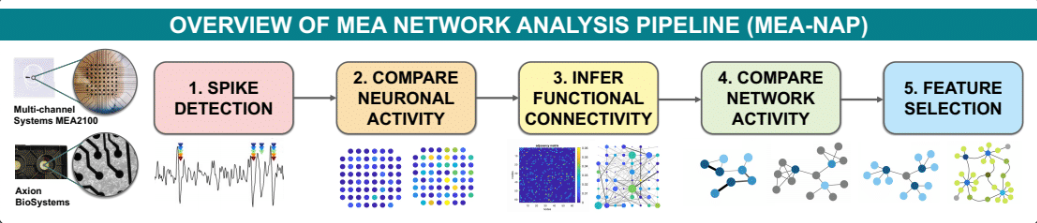
We have created two computational pipelines for analysing microscale functional networks. These pipelines brings together methods from many areas of network science including methods used to analyse regional or whole brain networks. With the MEA network analysis pipeline (MEA-NAP), we apply these metrics to analyse our MEA recordings.
Our paper describing MEA-NAP and its applications is now published in Cell Reports Methods!
MEA-NAP: A flexible network analysis pipeline for neuronal 2D and 3D organoid multielectrode recordings
Timothy PH Sit, Rachael C Feord, Alexander WE Dunn, Jeremi Chabros, David Oluigbo, Hugo H Smith, Lance Burn, Elise Chang, Alessio Boschi, Yin Yuan, George M Gibbons, Mahsa Khayat-Khoei, Francesco De Angelis, Erik Hemberg, Martin Hemberg, Madeline A Lancaster, Andras Lakatos, Stephen J Eglen, Ole Paulsen, Susanna B Mierau. Cell Reports Methods, 4(11), November 18, 2024. https://www.cell.com/cell-reports-methods/fulltext/S2667-2375(24)00291-1
Our manuscript is also available at PubMed Central:
MEA-NAP compares microscale functional connectivity, topology, and network dynamics in organoid or monolayer neuronal cultures.
Timothy PH Sit, Rachael C Feord, Alexander WE Dunn, Jeremi Chabros, David Oluigbo, Hugo H Smith, Lance Burn, Elise Chang, Alessio Boschi, Yin Yuan, George M Gibbons, Mahsa Khayat-Khoei, Francesco De Angelis, Erik Hemberg, Martin Hemberg, Madeline A Lancaster, Andras Lakatos, Stephen J Eglen, Ole Paulsen, Susanna B Mierau
bioRxiv 2024.02.05.578738
https://pmc.ncbi.nlm.nih.gov/articles/PMC10871179/
We are also developing CAT-NAP, our calcium imaging network analysis pipeline in collaboration with scientists at MIT.
Combining cellular-scale neuronal network recordings with spatial transcriptomics
This interdisciplinary research involves the intersection of
neurophysiology, single cell genomics, human stem cell biology, and network science. We are addressing how genetic changes alter
neural network function during early development. Using iPSC-derived human cortical cultures, we now have the ability to understand
how network function is altered at the cellular scale in a human in vitro model of brain development. In this project, we will grow
human-derived neuronal cultures and record the neuronal network activity over early development using microelectrode arrays (MEA)
and calcium imaging. Through an exciting collaboration with the Hemberg group (BWH), Vanessa Jane Hall (U of Copenhagen, Denmark)
and Yannick Coffinier (U of Lille, France), our aim is to combine MEA recordings with spatial transcriptomics using MERFISH. This would provide
a novel platform for understanding how gene expression correlates with neuronal network activity and how these processes are altered
in neurological disorders. This work is supported by the Novo Nordisk Foundation.
Image: Schematic of how we can combine MEA recordings of network activity with post-recording spatial transcriptomics.
Currently recruiting team members for the following projects
Network dysfunction in human cellular models of autism spectrum disorder (ASD)
Using iPSC-derived neuronal cultures from people with genetic causes of ASD, we will compare the development of functional connectivity in the
cellular scale networks using MEA recordings and calcium imaging. One of the key questions is what network features multiple genetic causes of ASD,
for example those that alter synaptic NMDA receptor function, may share. We are looking for team member with and/or desire to develop expertise in
initiation and maintainance of neuronal cultures (2D and 3D), electrophysiology, imaging, and/or computational analysis.

Real-time detection of network properties from spontaneous activity
Using metrics from control theory and graph theory, we can identify nodes that have a larger influence on the overall network activity
observed than other nodes in the network. We are looking for team members with computer science, engineering and/or neuroscience backgrounds who are
interested in creating a real-time implementation of our network analysis pipeline during microelectrode array (MEA) recordings or calcium imaging. This
would allow us to first record network activity and once high and low influence nodes are identified to selectively stimulate these nodes--either with
electrical or optogenetic stimulation--to validate these predictions. This approach could also be used to test novel therapeutic strategies for restoring
network function in our cellular models of autism spectrum disorder and other neurologic disorders.
Published collaborations where we
contributed our electrophysiology and network expertise
Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, Lakatos A, Lancaster MA. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 2019 Apr;22(4):669-679.
Szebényi K, Wenger LMD, Sun Y, Dunn AWE, Limegrover CA, Gibbons GM, Conci E, Paulsen O, Mierau SB, Balmus G, Lakatos A. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat Neurosci. 2021 Nov;24(11):1542-1554.